2 8 8 16 Electron Shells
They both have _____.

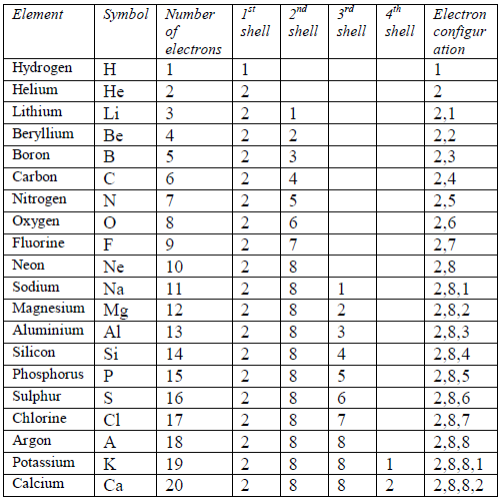
2 8 8 16 electron shells. They both have two electron shells. 2-8-8-1 has 4 shells. The number of electron a shell can have from the first to second shell is two then eight.
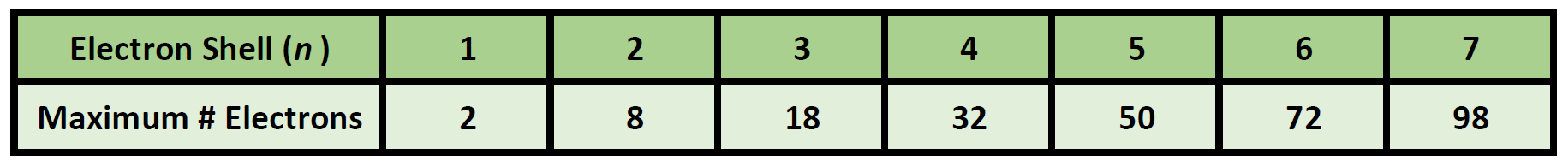
The maximum number of electrons in each shell, going from the middle to the outside, is 2, 8, 8, 18. Shell number one can only hold 2 electrons, shell two can hold 8, and for the first eighteen elements shell three can hold a maximum of eight electrons. Electron shells make up the electron configuration of an atom.
When this is filled, electrons go into the third shell, which also holds a maximum of eight electrons. The electrons like to be in separate shells/orbitals. Its electron structure is 2, 8, 8, 1.
The Bohr model was developed by Niels Bohr in 1913.In this model, electrons exist within principal shells. Lithium (Li) and oxygen (O) are both in period 2. Bonding in \(H_2\) and methane (\(CH_4\)) The other tendency of atoms is to maintain a neutral charge.
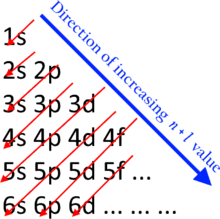
One More Thing Most elements can only use electrons from their outer orbital to bond with other elements. 1s2 2s2 2p x 1 2p y 1 2p z 1 2nd shell needs 3 electrons Forms 3 covalent bonds O:. As more electrons are added, higher energy levels with more sub-shells become filled.
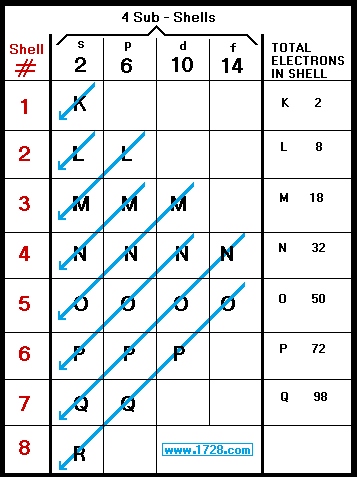
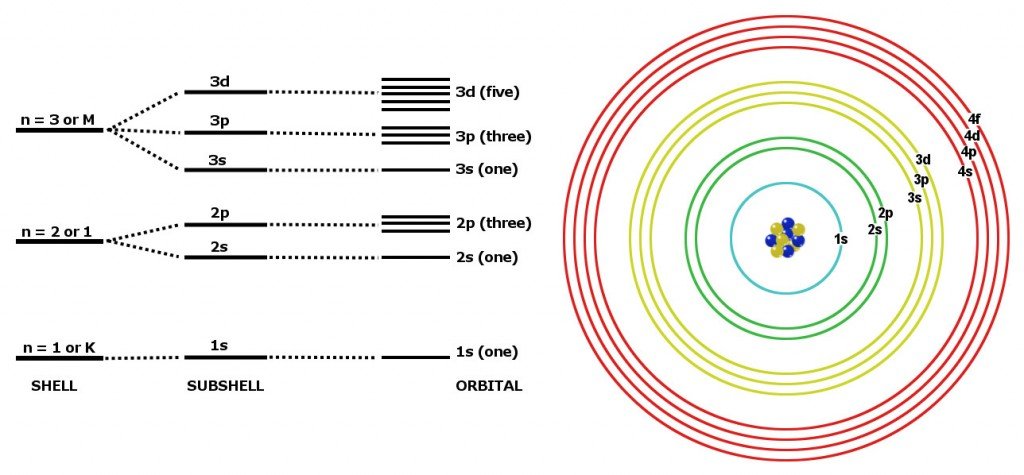
The M shell contains 3s, 3p, and 3d, and can carry. Can't believe I always used to write e.g electron configuration of calcium 2,8,8,2. After looking closely it actually relates to the electron config.
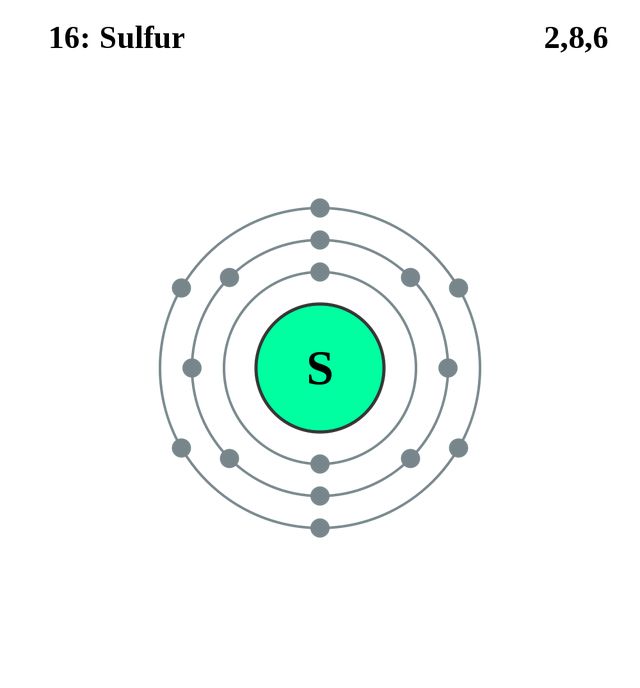
If we were to distribute electrons unconsciously with respect to how the sub-shells are lined up, as shown in the figure above, Calcium (Ca) with atomic number would have the configuration 2,8,10 (2, 2+6, 2+6+2). The electron configuration (electronic configuration) of an atom of sulfur is 2,8,6 An atom of sulfur has 2 electrons in the first energy level (K shell), 8 electrons in the second energy level (L shell) and 6 electrons in the third energy level (M shell). The electron configuration of this is 1s2 2s2 2p6 3s2 3p6 (2+2+6+2+6=18) 18 is the atomic number of 18Ar or.
After filling the first shell level (with just an s subshell), electrons move into the second-level s subshell and then into the p subshell before starting on another shell level. This means that the first energy level (the K-shell) contains 2 electrons, both in sub-shell s, and that the second energy level (the L-shell) contains 6 electrons, 2 in sub-shell s and 4 in sub-shell p. So it has 6 outer shells.
This is because it is the maximum capacity of the 3rd shell and it doesn't tell about the order in which the electrons are filled. The arrangement of electrons can also be shown using a 'Dot-and-cross' diagram. The 8 stands for the 2s2 and 2p6.
This page contains materials for the session on the electron shell model and quantum numbers. The electron configuration is Ne3s 2 3p 3. Subsequent shells can hold more electrons, but the outermost shell of any atom holds no more than eight electrons.
The electronic structure of each element can be shown simply as the number of electrons in each shell. This decides the electron capacity of the shells. In an atom, the electrons spin around the center, also called the nucleus.
Then the fourth shell begins to fill. _____ FILL IN THE ELECTRONS. Oxygen's electron configuration is:.
Log in for more information. How many shells do 2 electrons need?. Choose from 500 different sets of term:electron shells = 2, 8, 8 flashcards on Quizlet.
You can write the full electron configuration in terms of subshells. An electron normally exists in the lowest energy shell available, which is the one closest to the nucleus. For example, a sodium atom, Na, has a single electron in its valence shell, surrounding 2 stable, filled inner shells of 2 and 8 electrons.
Electron Shells and the Bohr Model Figure \(\PageIndex{1}\):. And lastly the 2 standing for 4s2. Enjoy the videos and music you love, upload original content, and share it all with friends, family, and the world on YouTube.
After that i've gotten so many differing answers and explanations i have no idea which is right. The 2–8–8 Rule is a rule in general chemistry that states that the first valance shell can hold 2 valence electrons, the second one can hold 8, and the third one can hold 8. The shells can also be called energy levels.
And the third 8 stands for 3s2 and 3p6. 16 electrons 16 shells. For example, lithium is 2.1, neon is 2.8.8, and calcium is 2.8.8.2.
" 2 xx "4 orbitals" = "8 electrons" "M:. 100 Fm (Fermium) Legend_color:#ff99cc 2,8,18,32,30,8,2 Fm 2,8,18,32,30,8,2 Fm. 8 electrons 8 shells.
That is certainly possible, and in fact very likely. The remaining electron will appear in the second shell in the 2s subshell. " 2 xx "1 orbital" = "2 electrons" "L:.
Then draw them in their proper shells. There are two shells in period 2 (that's why it's named like that)The first can hold (max.) 2 electrons (1s2), the second holds 2+6 = 8 electrons (2s2 and 2p6) at maximum. The maximum electrons that can be carried by the sub-shell S is 2, by P is 6, by D is 10, and the F sub-shell can carry 14.
An up-to-date periodic table with detailed but easy to understand information. Learn term:electron shells = 2, 8, 8 with free interactive flashcards. This means that each energy shell can hold a maximum of "K:.
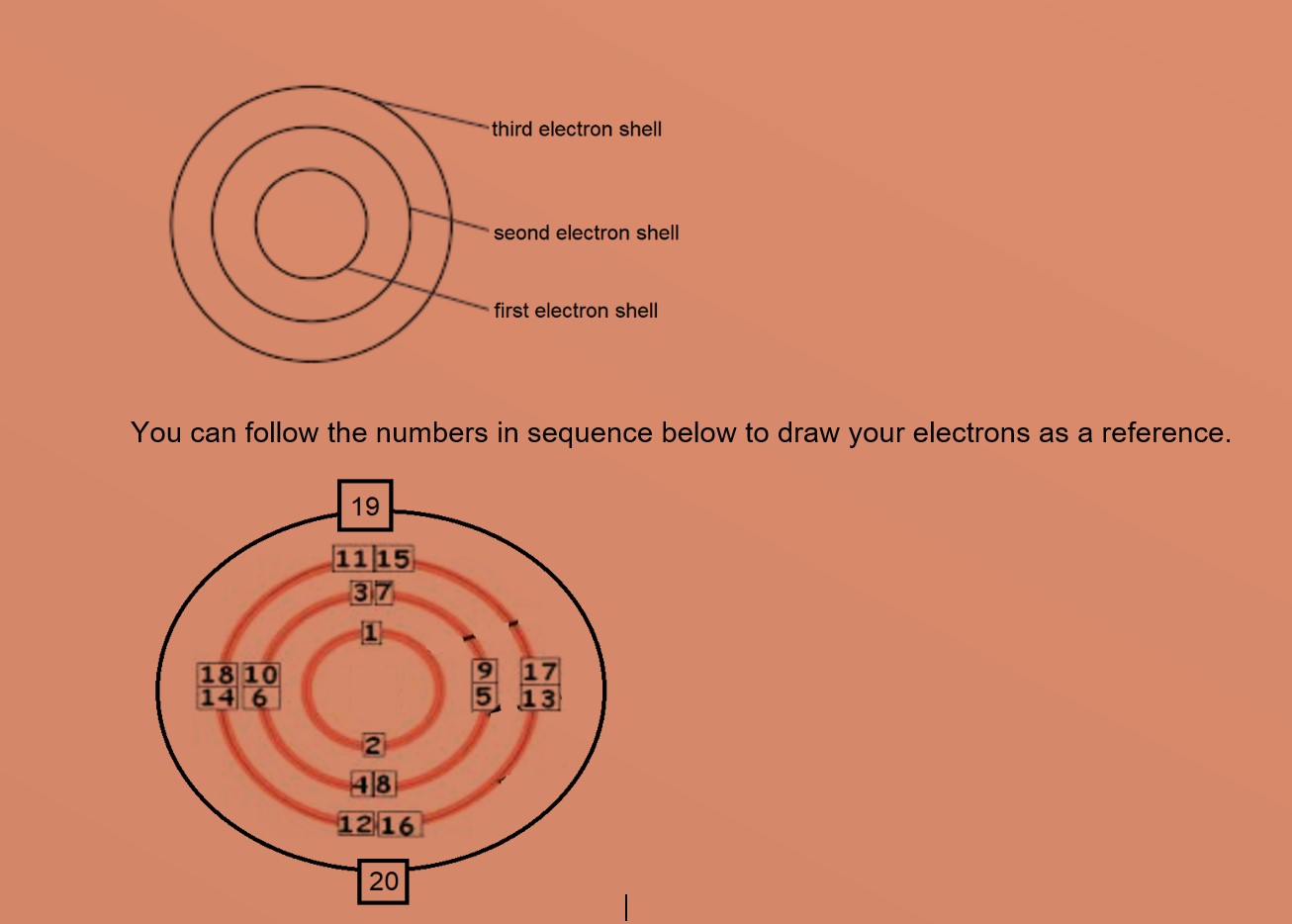
Electron shells are drawn as circles, with the electrons on each shown as dots or crosses. Are you making a model of an atom and need to know how to place the electrons around the nucleus?. We will use the term shell rather than energy level but either is acceptable.
14 electrons 14 shells. Below is a picture of a potassium atom. 2) Orbitals are combined when bonds form between atoms in a molecule.
103 Lr (Lawrencium) Legend_color:#ff99cc 2,8,18,32,32,8,3 Lr 2,8,18,32,32,8,3 Lr. 2s 2 2p 4:. Asked 24 days ago|10/9/ 6:52:11 PM.
The second shell can hold a maximum of eight electrons. Nuclear physics predicts an island of stable elements around an atomic number of 1 $^{1}$, with the two heavier of the three elements predicted to be most stable having an $\ce{8s^2}$$^{2}$ and an $\ce{8s^2 8p^1}$$^{3}$ configuration, respectively. Within each shell of an atom there are some combinations of orbitals.
Learn vocabulary, terms, and more with flashcards, games, and other study tools. For example, an atom with 6 of 8 electrons in its outer shell will try to gain 2 electrons so its outer shell is full. The Bohr model, which is a semi-classical model of an.
So if you're looking at oxygen at 8, its code is 2s2,2p4. Look on the periodic table under the symbol for the electron configuration. 2-8-8-8-1 had 5 shells.
Ionic Bonding Ionic bonding occurs when one element donates an electron (or electrons) to another so that both elements will have a full outer shell. Only the noble gases (the elements on the right-most. How many shells do 8 electrons need?.
How many shells do 18 electrons need?. If so, you w. Going back to the above example, Lithium is 1s 2 2s 1 (1s has 2 electrons, 2s has 1 electron).
This is why the hydrogen atom has an electron configuration of 1s 1. In your case, the element is said to contain 2 electrons on its first shell, 8 electrons on its second, and 8 electrons on its third. 1s2 2s2 2p x 1 2p y 1 2p z 0 2nd shell needs 4 electrons Forms 4 covalent bonds N:.
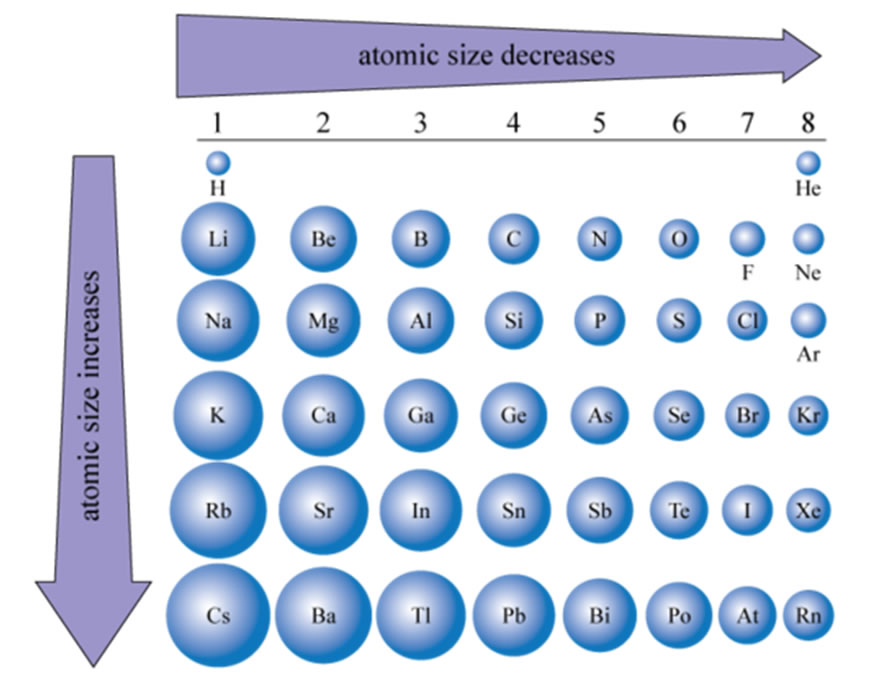
Normally two electrons pairs up and forms a bond, e.g., \(H_2\) For most atoms there will be a maximum of eight electrons in the valence shell (octet structure), e.g., \(CH_4\) Figure 1:. The same number of protons two electron shells the same number of electrons similar properties. The 2,8,18,… configuration is taught till class 10th in schools.
Since these filled shells are very stable, a sodium atom tends to lose its extra electron and attain this stable configuration, becoming a sodium cation in the process Na → Na + + e − On the other hand. How do I read an electron configuration table?. Because of its lower energy state, the 4s orbital fills before the 3d, and later s orbitals fill similarly (for.
Figure out how many electrons each of these atoms have. 8 electrons occupy the second shell 1 electron occupies the third shell This electron arrangement can be written as 2.8.1 (each dot separates one shell from the next). 1s1 1st shell needs 1 electron Forms 1 covalent bond C:.
How many shells do 16 electrons need?. In Bohr's electron configuration, can the energy shells hold a 2, 8, 8, 18, or 2, 8, 18 maximum number of electrons?. And i'm pretty sure its 18 then 32 after that.
Transition metals can use the two outermost shells/orbitals to bond with other elements. In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's nucleus.The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus.The shells correspond to the principal quantum numbers (n. How many shells do 4 electrons need?.
The total number of electrons in an atom of sulfur = 2 + 8 + 6 = 16. Any high school chemistry textbook will tell you that this isn’t correct, as the precise configuration is 2,8,8,2. The outer shell contains too many electrons 2) There should be two electrons in their inner shell.
More detailed versions of the periodic table (you can find an excellent example here) often show the electron configuration as a comma-separated list of values showing the number of electrons in each shell.For example, silicon (Si) would have the electron configuration 2, 8, 4.Electron shells 1n and 2n are full, containing two and eight electrons respectively, while electron shell 3n contains. Are the electron shells 2, 8, 18, 32, 18, 8, 2?. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.
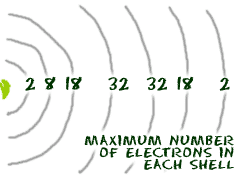
This gives a valence-electron configuration of 3s 2 3p 3. The name for electron shells comes from the Bohr model, in which groups of electrons were believed to go around the nucleus at certain distances, so that their orbits formed "shells". No shell can have more than 32 electrons.
C We obtain the valence electron configuration by ignoring the inner orbitals, which for phosphorus means that we ignore the Ne closed shell. There are four types of orbitals that you should be familiar with s, p, d and f (sharp, principle, diffuse and fundamental). Orbit in shells of 2 8 8 18etc Electrons in outermost shells interact Quick from GEO 303 at University of Texas.
2 electrons 2 shells. Electrons fill in shell and subshell levels in a semiregular process, as indicated by the arrows above. The number of electrons that can be in a shell is equal to math2n^2/math.
Atoms will tend to react in such a way to obtain the valance shell config. Start studying Electron Shells. I assume you mean that orbital 1 has 2 electrons, orbital 2 has 8 and orbital 3 has 8.
The outermost shell is always the # of elements in that elements row. The electron shell configurations for 29 of the first 36 elements are listed in Table 2.2. The answer to your question lies in knowing the rea.
Hope that helps :) 0 0. Orbitals in the Bohr model:. Each of those colored balls is an electron.
" 2 xx "9 orbitals" = "18 electrons" And so on. The 2 stands for the 1s2. Shown at AS e.g 1s2, 2s2, 2p6, 3s2, 3p6 4s2.
You will find it's usually 2, 8, 18 or 32 for the maximum number of electrons in an orbital.

Periodic Table Position And Electron Configuration Introduction To Chemistry

Slides Show

I My Chemistry Book Electron Configuration Of Of Iron Is 2 8 14 2 But By Using Formula Of 2 Brainly In
2 8 8 16 Electron Shells のギャラリー

Electron Shell Diagrams Of The 118 Elements

Topic 1 Electron Shells 1st Shell 2 Electrons 2nd Shell 8 Electrons 3rd Shell 8 Electrons Science Chemistry 7th Grade Science Science

Class Copy The Periodic Table Review Sheet Directions Answer
Electron Shell Wikimedia Commons

Name Date P R S Honors Chemistry Electron

How Are Electrons Distributed In Different Orbits Electronic Configuration

Energy Of Closed Shell H 8 With A Minimal Basis Set With 0 2 4 6 8 Download Scientific Diagram
Electron Configurations

Atomic Structure Nickel

Electron Shell Diagrams Of The 118 Elements
How To Find The Group Number And Period Number When The Electronic Configuration Is Given Quora

Atomic Structure Nucleus Proton Neutron Electron Mass Charge Isotopes Electron Arrangement Rutherford Bohr Model Of Atom Allotropes History Of Atomic Structure Model Development Ionisation Ions Gcse Chemistry Revision Notes Quizzes Ks4 Science

Chemistry The Atom Basic Structure 2 C Sser Ltd Ppt Download

Claes Johnson On Mathematics And Science Quantum Contradictions 9 Shell Structure From Helium Scaling

The Atomic Regular Polyhedron Electronic Shell
Q Tbn 3aand9gctpd3jefdq2cbjdrgnoyp68em 3retaocdesxpal6mhb7fzoakv Usqp Cau

Shell Atomic Model Physics Britannica

Structure And Bonding Title Atomic Structure Aim To Draw And Label The Sub Atomic Particles Of An Atom Ppt Download
Chem4kids Com Elements Periodic Table Transition Metals
Ionic Compounds Manoa Hawaii Edu Exploringourfluidearth
:max_bytes(150000):strip_icc()/Nickel-58b602195f9b5860464c3e1d.jpg)
Atom Diagrams Electron Configurations Of The Elements
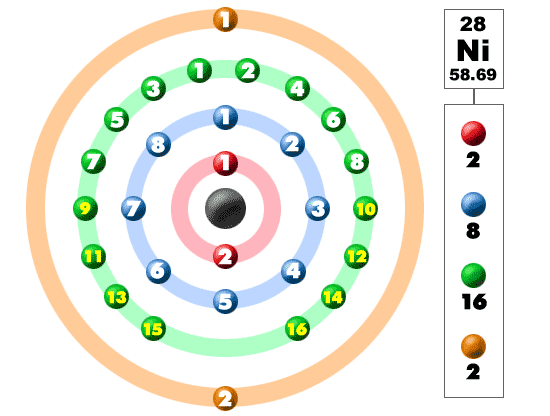
Chem4kids Com Nickel Orbital And Bonding Info

Valence Electron Wikipedia

O Level Chemistry 05 15 13

Electron Shell Wikipedia
Www Newpaltz K12 Ny Us Cms Lib Ny Centricity Domain 812 4 atoms and periodic table ws 19 with class notes and key Pdf

S Sulfur Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

C1 1 Atom Dot Electron S And Nucleus Diagrams Secondary Science 4 All
Electron Shells

Quantum Structure Of Glycine 30 Orbitals Subshells 15 Electron Download Scientific Diagram
Http Schools Birdville K12 Tx Us Cms Lib2 Tx Centricity Domain 727 Review 8 5b atomic structure and chemical properties Pdf

Core Electron Wikipedia
Atomic Structure
Electronic Arrangements Maktaba Elearning
Http Mdep Moe Edu Mm Dbebox Public Pdf G10text Chem Unit3 Exercises Pdf

What Is The 2 8 8 Rule What Is It Used For Quora
Electron Shell Wikipedia
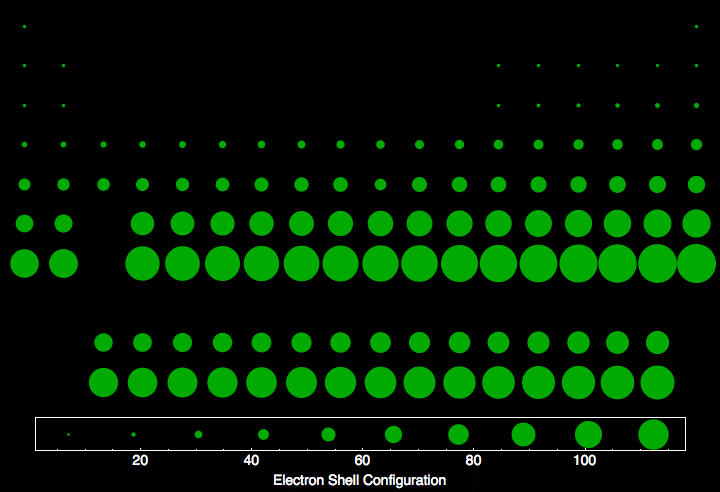
Electron Shell Configuration For All The Elements In The Periodic Table

Electron Configurations For The Third And Fourth Periods Video Khan Academy
Chemistryklipz Files Wordpress Com 17 01 Igcse Atoms And Pertab Wpress Pdf

In The Periodic Table Why Doesn T The 2nd Row Have Exactly 2 Elements Chemistry Stack Exchange

Solved The Figure Below Indicates The Electrons In The Fi Chegg Com

New Simplified Chemistry Class 9 Icse Solutions Atomic Structure A Plus Topper
Ch150 Chapter 2 Atoms And Periodic Table Chemistry

How Are Electrons Distributed In Different Orbits Electronic Configuration

How Are Electrons Distributed In Different Orbits Electronic Configuration

Ni Nickel Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

Essentials Of Radiographic Physics And Imaging 1st Edition Johnston T
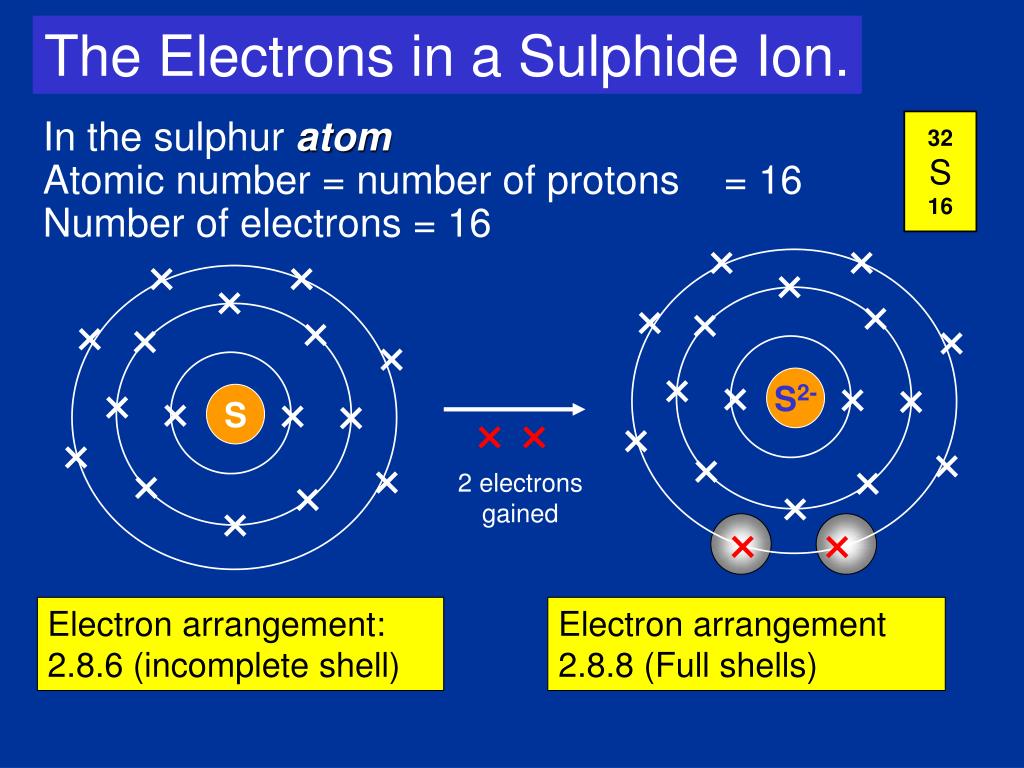
Ppt Ionic Bonding L O Powerpoint Presentation Free Download Id
Question C9d7f Socratic

I Thought In The Third Shell There Are 18 Electrons But Why Are They Gaining Only 2 Electrons Pls Brainly Com
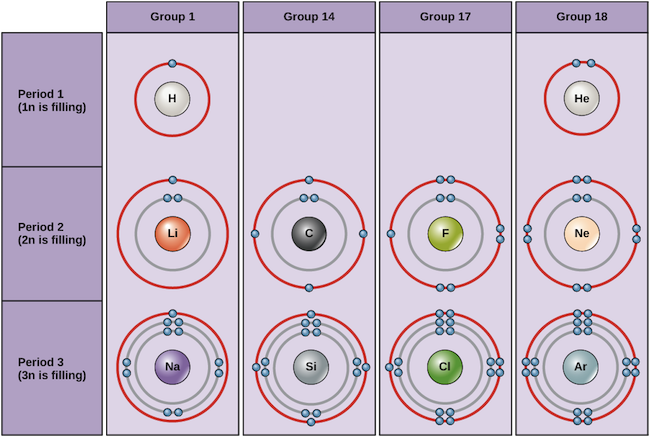
The Periodic Table Electron Shells And Orbitals Article Khan Academy

If Copper Has 2 Valance Electrons And Sulfur 6 Why Don T They Bind In Pairs Chemistry Stack Exchange
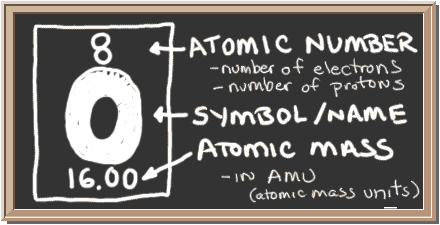
Chem4kids Com Oxygen Orbital And Bonding Info

6 4 Electronic Structure Of Atoms Electron Configurations Chemistry
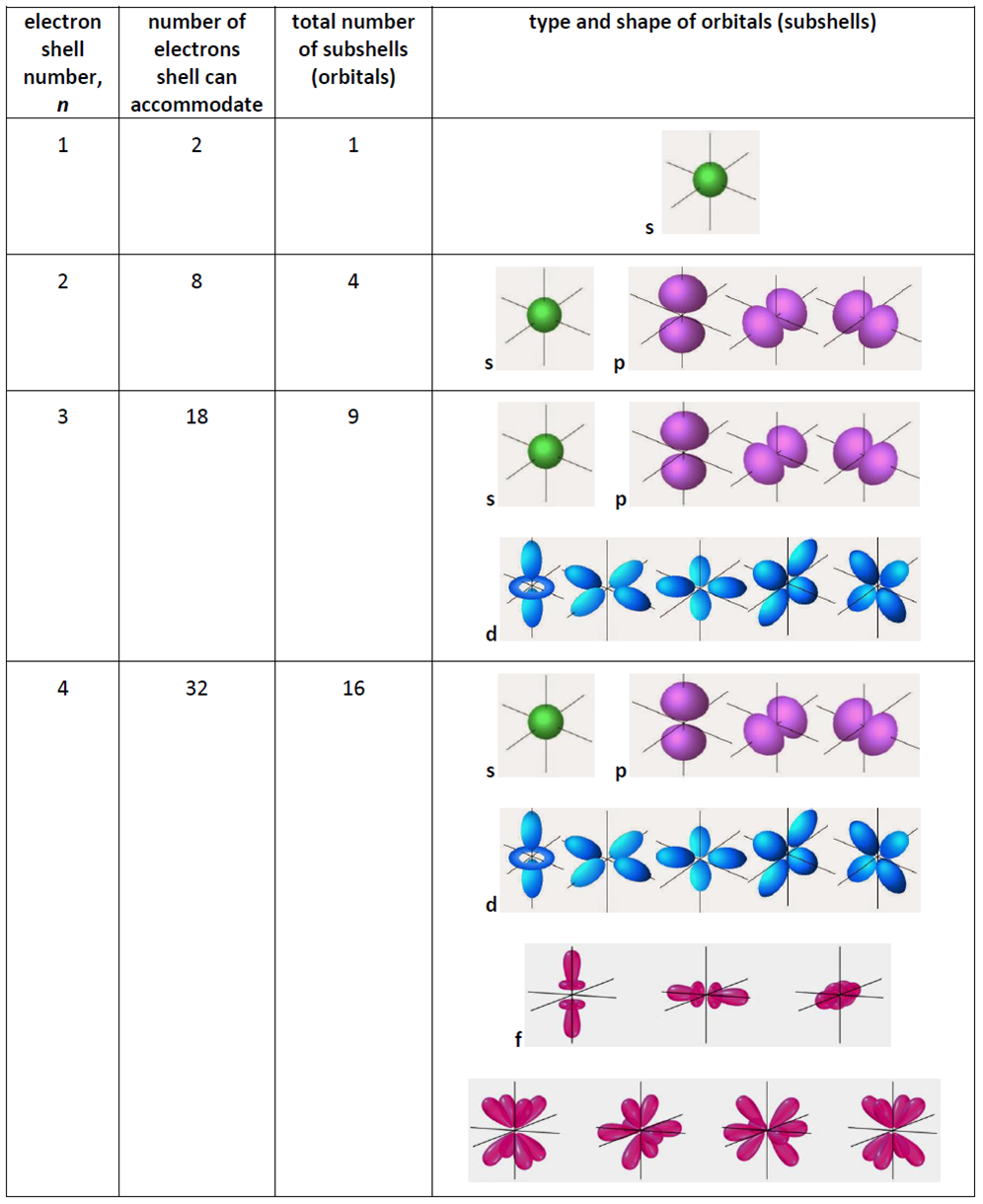
V2 3

Distribution Of Electrons In Different Orbits Ck 12 Foundation

Solved 10 How Big Are Atoms 1 5 Um 0 1 0 5 Um O 1 5 Nm Chegg Com

Human Biology Online Lab Sulfur Madeleine Miller

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

परम ण और अण भ ग 2 परम ण क रम क क य ह What Is Atomic Number Electron Configuration Hindi Youtube

Chemistry The Atom Basic Structure 2 C Sser Ltd Ppt Download

Electron Shell Wikimedia Commons

Electron Shell Wikipedia

Periodic Table The Periodic Table Britannica

Electron Configuration Chemistry Socratic

Shedding Light On Atoms Episode 6 Electron Shells Liacos Educational Media

Ds Darmstadtium Element Information Facts Properties Trends Uses And Comparison Periodic Table Of The Elements Schoolmykids

Bohr Models Are Not Boring How To Draw Bohr Diagrams Ppt Download
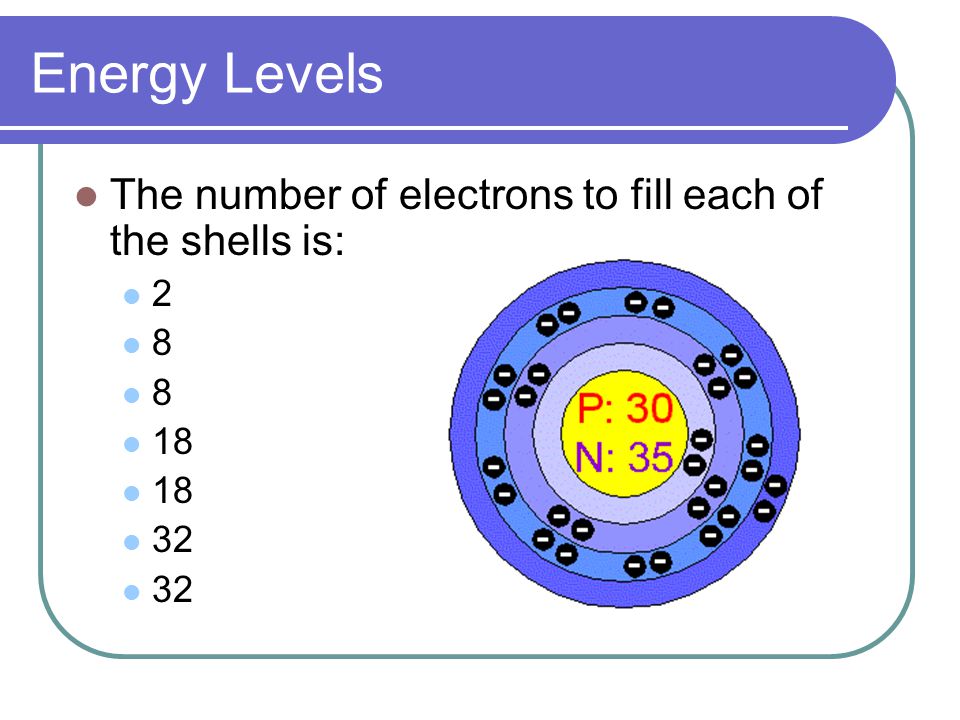
The Bohr Model And Electron Dot Diagrams Ppt Video Online Download
Q Tbn 3aand9gcq3qyc7vgotow 7horwirxv7 F1jp 4natjs6fcnpytlk87dmub Usqp Cau

The Atoms Family Cheat Sheet By Phoebe12 Download Free From Cheatography Cheatography Com Cheat Sheets For Every Occasion

Potions For Muggles Revisiting The Bohr Model
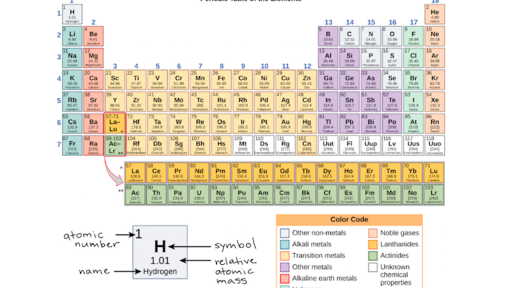
The Periodic Table Electron Shells And Orbitals Article Khan Academy
Www Immanuelcollege Net Wp Content Uploads 18 03 Y8 Bonding Structure And Properties Higher Pdf

Number Of Valence Electrons In Silicon

Hi Friends In The Above Picture You Can See Some Elements And Their Electronic Configuration In M Brainly In

Solved A 1 B 10 C 8 D 2 How Many Electrons Will A Sin Chegg Com
1

Shedding Light On Atoms Episode 6 Electron Shells Liacos Educational Media
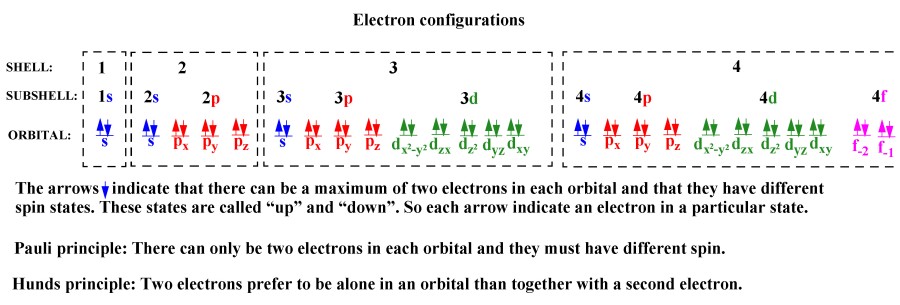
12 1 Electron Configuration
Electron Shells And Orbitals

Atomic Structure Online Presentation

Physical Origin Of Chemical Periodicities In The System Of Elements In Pure And Applied Chemistry Volume 91 Issue 12 19
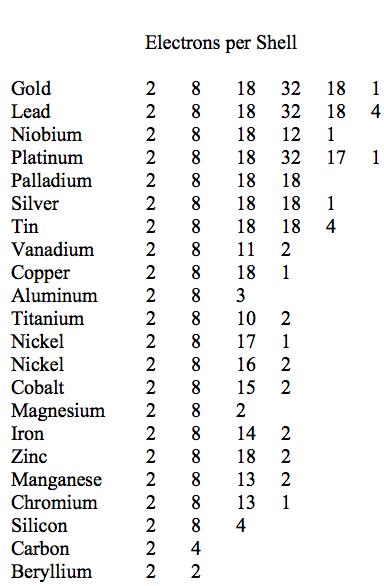
Physical Ductility Of The Elements Failurecriteria Com
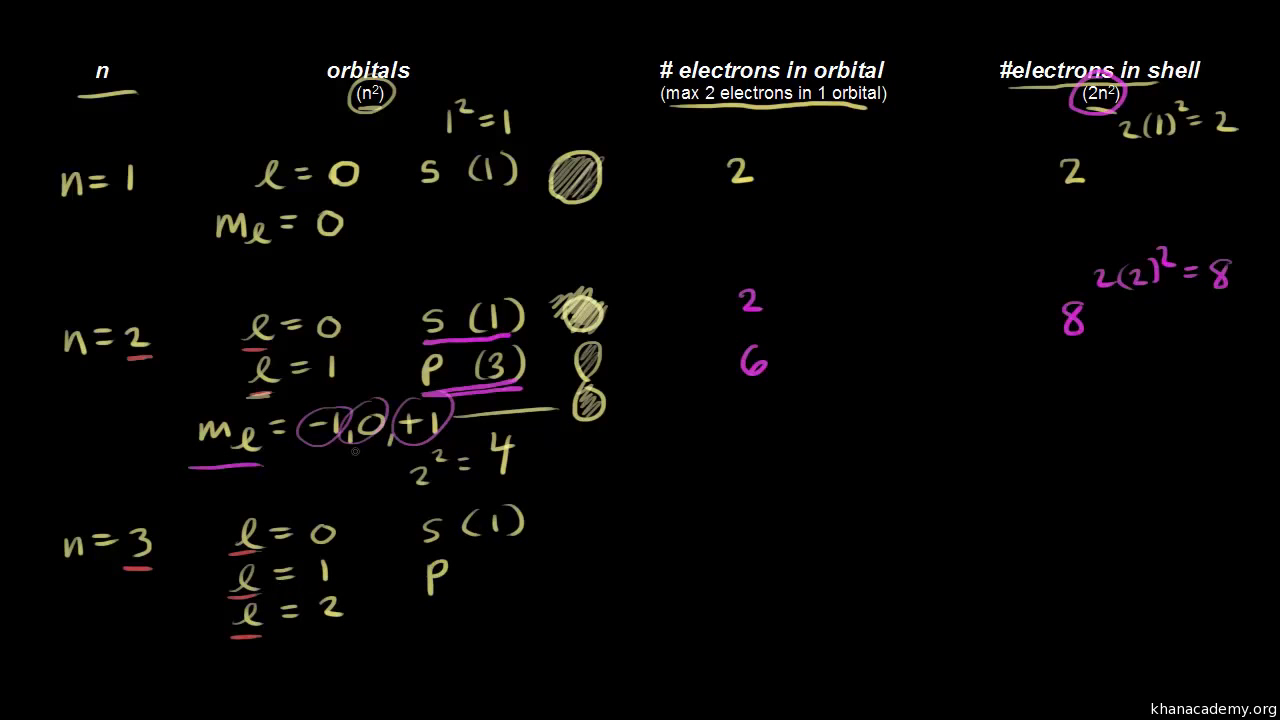
Quantum Numbers For The First Four Shells Video Khan Academy

How Are Electrons Distributed In Different Orbits Electronic Configuration
Why Don T We Put 18 Electrons In A Third Shell As It Has The Capacity Of 18 Electrons Quora

Ppt Ionic Bonding L O Powerpoint Presentation Free Download Id
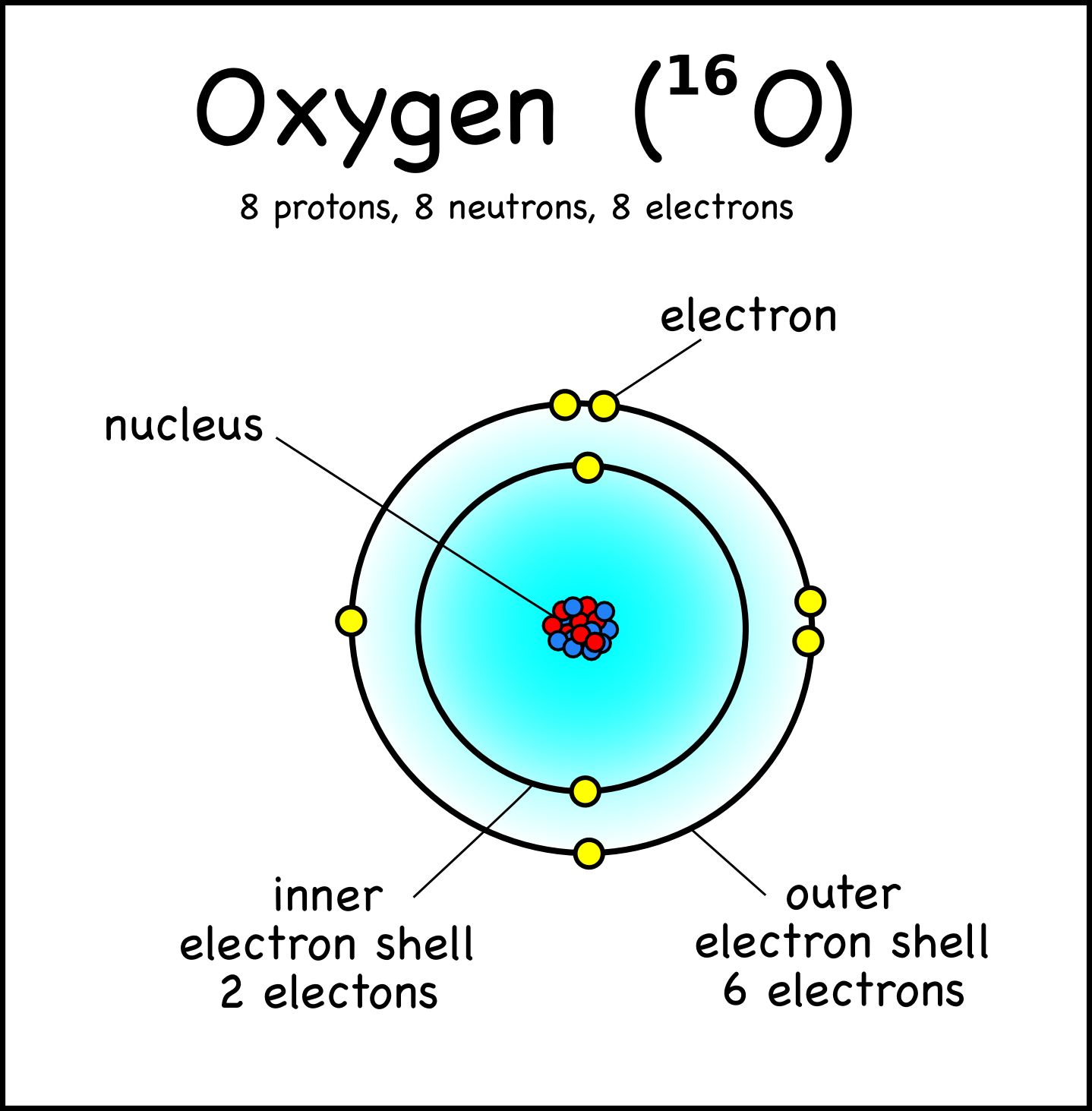
Question E9926 Socratic

Shedding Light On Atoms Episode 6 Electron Shells Liacos Educational Media

Atomic Structure
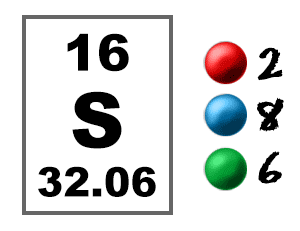
Chem4kids Com Sulfur Orbital And Bonding Info

Chemistry The Atom Basic Structure 2 C Sser Ltd Ppt Download
1
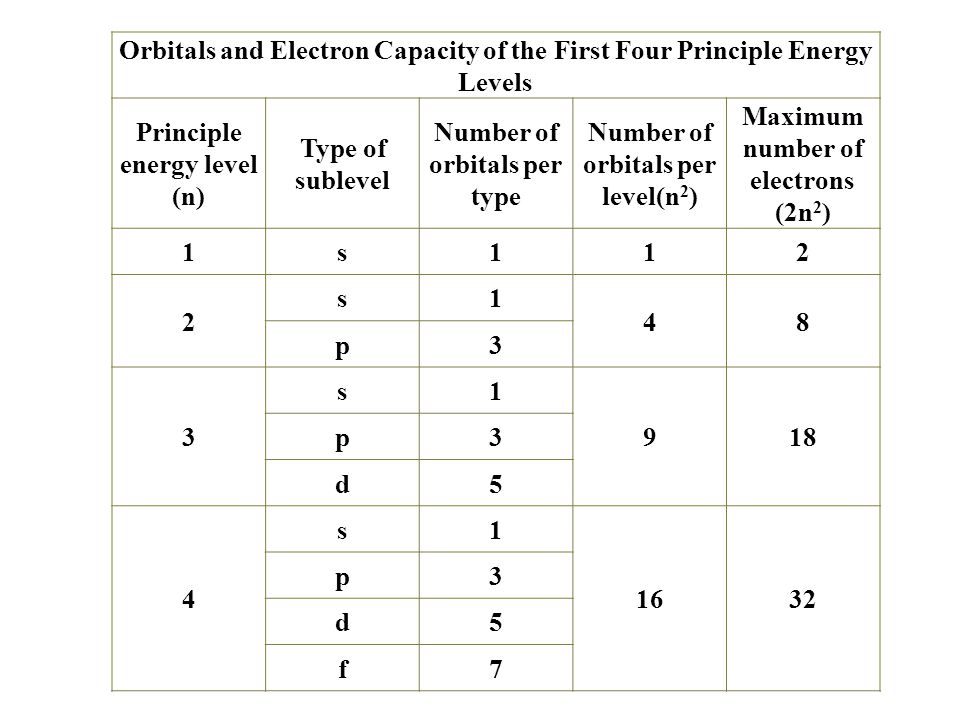
Electron Configurations

Solved How Many Electrons Fit Into Each Of The Lowest Ele Chegg Com

File Electron Shell 080 Mercury Svg Wikimedia Commons

Periodic Table The Periodic Table Britannica
What Are Valence Electrons And How To Find Them Where Are They Located

Atoms And Chemistry



